Our friend Amy, from Drug Development Wednesday, is back talking about CFRD. This article was originally published in CF Roundtable®, Summer 2018 newsletter.
Cystic Fibrosis Related Diabetes (CFRD) is the CF complication that I feared my whole life. I love sugary foods and I hate needles, so CFRD was definitely something I always wanted to avoid no matter what! I felt such a sense of ease every year when I passed my Oral Glucose Tolerance Test (OGTT) meaning I didn’t have CFRD. To further reassure me, my A1C was around 6.5-7, which according to my CF team, also confirmed I was in the clear. Life was good!
From 2010 to 2012, when I was ages 29-31, I started to feel so exhausted that I could barely get off the couch after work and on weekends. My FEV1 was high, but I expected that as I got older, CF would simply just take its toll and make me more fatigued. Over the years, I found myself sacrificing social time with friends, sacrificing time for hobbies, and even sometimes sacrificing time for self-care more and more often– all so I could sleep more. I knew I had to work to pay for my rent, food and medical bills, so I powered through my 40 hour/week job and slept where I could.
With the little spare time I did have in between work and sleeping, I did research online: I read scholarly articles about CF, I chatted with other patients with CF who had been through the various progressions of the disease, and of course, I constantly questioned my CF team for solutions. Thyroid problems? Hormone imbalance? Change in sputum cultures? Elevated white blood cell count? Elevated CRP? IgE? Nope. All was well!
Enter a new nurse to my CF team that suggested that I try to test my blood sugars on my own with a meter. Not my favorite idea because of my fear of needles and blood, but I was so desperate for answers after 2 years of such fatigue, I would try anything. What would you know? I was getting blood sugars in the 180s, 190s and low 200s mg/dL 2 hours after eating…. sometimes. For reference, anything above 120 mg/dL is considered to be elevated blood glucose (hyperglycemia). But the elevated sugars didn’t happen all the time – even when I would eat the exact same meal (or dessert J ).
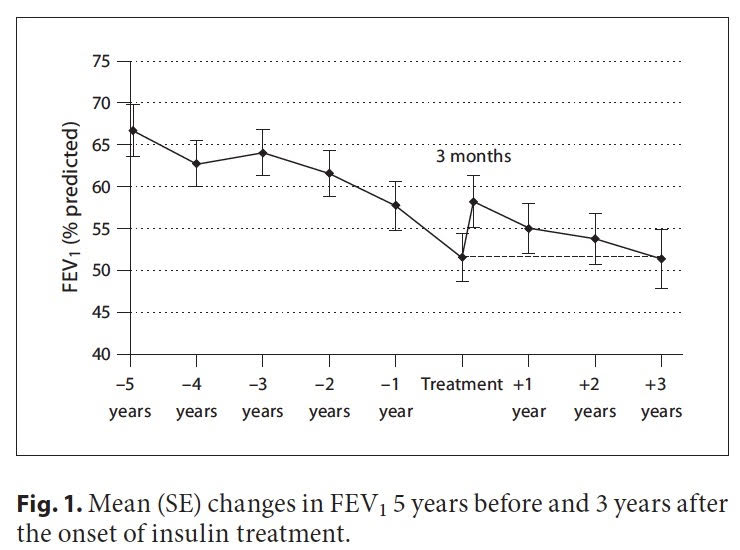
Off to the Endocrinologist where I learned that I had caught CFRD quite “early” – early enough to where my pancreas was working in fits and starts – meaning my precious pancreas would sometimes release enough insulin to cover what I was eating, but other times it simply just didn’t. I was put on long acting insulin (basal insulin) and almost overnight I felt like I gained my life back; it’s no exaggeration that even lowering my sugars slightly reduced fatigue dramatically. To say I was ecstatic would be a complete understatement.
I plugged along for the following months, tracked my blood sugars several times a day with a meter, recorded what I ate, counted those fun carbs and sugars that I LOVE, and what activity I did during the day and when. It was quite a bit of work, but it was so incredibly worth the effort because my new energy level made me feel like a new woman! Eventually my tracking led me to initiate fast-acting insulin depending on what I ate and I learned the in’s and out’s of how my body reacted to exercise, heat, different foods, and time of day.
All this is to say that being diagnosed with CFRD at age 31 was quite the adventure – but I have learned over the years my situation wasn’t that atypical. Sadly, the standard diagnosis techniques for CFRD may be missing some patients. Here’s why that matters:
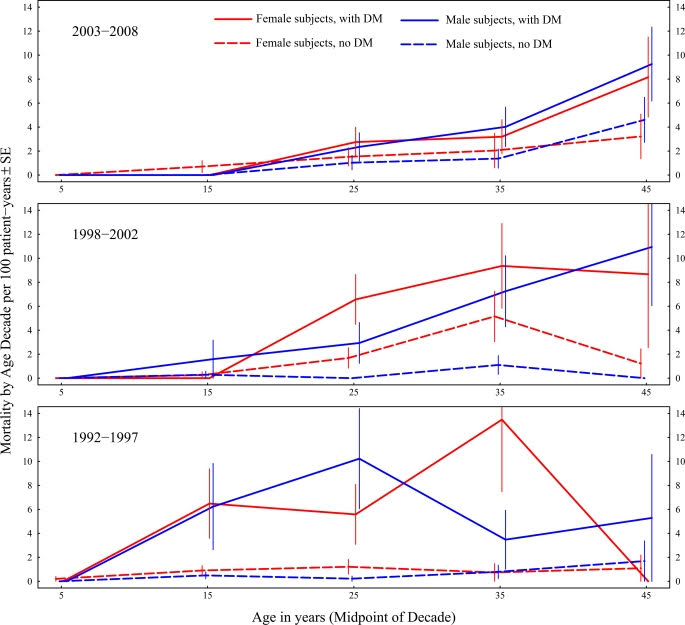
According to retrospective studies including one by Rolon MA, etc al, “the years preceding therapy for CFRD, reductions in FEV1, forced vital capacity (FVC), and body mass index (BMI) are observed.” [1] Meaning that before a person with CF initiates therapy, FEV1 can decline – the best predictor we have of health in a person with CF. In fact, as early as 1988, it was reported that less than 25% of CF patients with diabetes reached the age of 30 compared with about 60% of those without diabetes. [2] Dr Moran et al. that earlier diagnosis and treatment of CFRD decreased mortality over 20 years. Let that sink in – CFRD may really have an impact on lung health – but unlike me, a person with CF may not feel any symptoms of CFRD, particularly in the early stages.
What’s more concerning is that current guidelines suggest utilizing diagnostic criteria that may miss the presence of CFRD, let alone pre-diabetes. The Cystic Fibrosis Foundation’s CFRD Guidelines from 2010 recommend an annual Oral Glucose Tolerance Test (OGTT) for CFer’s over the age of 10 – and a diagnosis of diabetes occurs when blood glucose 2 hours after initiation of the test is ≥ 200 mg/dL (11.1 mmol/L)[3] An OGTT involves the patient drinking a high sugar drink – and glucose and insulin levels are measured typically at 1 and 2 hours post consumption. According to María Clemente León et al, an OGTT may very well miss hyperglycemic episodes and a Continuous Glucose Monitor (a small device that is worn on the body that measures blood sugar levels every few minutes) more frequently detects these episodes.[4] Which is so fascinating for me to read because I definitely fell in to the category of an OGTT missing my elevated blood sugar episodes – but that didn’t mean they weren’t happening. I wish I had known this was possible in the years prior to my diagnosis! Let’s summarize: not only can CFRD cause lung function decline, but diagnosis can be missed in the CF population because of tools that are recommended in diagnosis.
The literature seems to be clear, as verbalized by Mainguy C et al in 2016: “The conventional OGTT, however, has been shown to have weak capacity to diagnose [CFRD] in CF individuals as the inherent variability of the test and the variability observed in individual CF patients over time, means it does not accurately reflect glucose handling” [5]
What’s more, the threshold for diagnosis may need to be updated based on some new research since the 2010 guidelines were published by the CFF. The Foundation says a patient has CFRD if his or her 2 hour OGTT blood glucose is above 200 mg/dL. In a study on CFRD by Brennan AL et al, showed that not only did glucose appear in CF patients’ airways when blood glucose reached above 140 mg/dL, but this glucose in the lungs fueled bacteria growth. [6] Perhaps diagnostic guidelines should be reconsidered so that we’re not fueling bacterial growing in patients’ airways?
Where did 200 mg/dL for CFRD diagnosis even come from? “The cut-offs defining CFRD were taken from Pima Native Americans with Type 2 Diabetes, in which they were used to forecast microvascular complications (e.g. retinopathy) and may not be appropriate to use in CF,”[7] states Hameed S, et al. So really, the diagnostic criteria for CFRD doesn’t have much to do with CF patients at all – it was set by the American Diabetes Association to reduce microvascular complications (damage to kidneys, eyes, heart, nerve disease), not lung function decline. I would strongly argue that we need to aim criteria that more closely aligns with what matters in CF – lung issues that lead to early death.
Lastly, those with CF and CFRD tend to have inhibited 1st phase insulin release[8] therefore, there can be a 30 minute or 60-minute spike in blood glucose after eating. This further demonstrates that a 2-hour glucose measure with OGTT may be completely missing hyperglycemic episodes – and therefore missing a diagnosis. In fact, I can recall getting low blood sugar episodes in my teenage years (12+ years before my diagnosis) as a result of blunted 1st phase insulin release after a carb heavy meal. I was getting an overly eager 2nd phase insulin response, which caused 2-3 hour post meal blood sugar lows – turns out this is a common early sign of decreased insulin a person with CF called reactive hypoglycemia.
“It may be advisable to begin insulin therapy early for people with or developing CFRD, as glucose tolerance declines, especially as CFRD is the result of progressive β cell dysfunction” states Kayani Kayani Et al. [9]
Given the unquestionable impact hyperglycemia has on cystic fibrosis patients, their lungs and prognosis, it is my hope that the Cystic Fibrosis Foundation will take into consideration the bountiful new research that has been published since 2010. At best, these current CFF 2010 CFRD Guidelines are preventing CFRD cases from being appropriately diagnosed, and at worst, the guidelines may be contributing to worsening lung function by many in our community – something we all simply cannot ignore. More large scale, multi-center research needs to be funded by the CFF to bring CFRD guidelines to reflect the latest research. Our lungs simply don’t have the luxury of waiting any longer. Looking forward, we are eagerly awaiting results of studies to see if using insulin in patients before they have CFRD will increase survival – stay tuned for those results hopefully soon!
I would like to thank Attain Health and Dr. Colleen Wood for opening my eyes to the latest research in CFRD. Without them, I would not have the slightest inclining about this space. In fact, you can access a webinar on CFRD here.
[1] Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubiana-Rufi N, et al. Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr (2001) 90(8):860–7. doi:10.1080/08035250152509555
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992212/
[3] https://www.cff.org/Care/Clinical-Care-Guidelines/Other-CF-Related-Conditions-Clinical-Care-Guidelines/Cystic-Fibrosis-related-Diabetes-Clinical-Care-Guidelines/
[4] https://www.sciencedirect.com/science/article/pii/S2530016417302380?via%3Dihub#bib0150
[5] Mainguy C, Bellon G, Delaup V, Ginoux T, Kassai-Koupai B, Mazur S, et al. Sensitivity and specificity of different methods for cystic fibrosis-related diabetes screening: is the oral glucose tolerance test still the standard? J Pediatr Endocrinol Metab (2017) 30(1):27–35. doi:10.1515/jpem-2016-0184
[6]Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, et al. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros (2007) 6(2):101–9. doi:10.1016/j.jcf.2006.03.009
https://www.cysticfibrosisjournal.com/article/S1569-1993(06)00093-2/fulltext
[7] Hameed S, Jaffé A, Verge CF. Advances in the detection and management of cystic fibrosis related diabetes. Curr Opin Pediatr (2015) 27:525–33. doi:10.1097/MOP.0000000000000251
[8] Lanng S, Thorsteinsson B, Røder ME, Orskov C, Holst JJ, Nerup J, et al. Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol (1993) 128(3):207–14. doi:10.1530/acta.0.1280207
[9] https://www.frontiersin.org/articles/10.3389/fendo.2018.00020/full#B18
The views expressed on any guest column, are that of guest contributors, and not necessarily those of Gunnar Esiason or the Boomer Esiason Foundation. Nothing in guest columns should be considered medical advice; such advice can only be given by a physician who is experienced with cystic fibrosis. The Boomer Esiason Foundation, Gunnar Esiason, and guests cannot be held responsible for any damage which may result from using the information on this website without the permission of your medical doctor.