Amy is back for the second installment for Drug Development Wednesday!
Welcome back! Our drug development story continues today with an in-depth dive in to the several phases of clinical trials (from pre-clinical to Phase IV) in the United States. This blog will discuss some highlights of each phase of drug development, including cost, time, number of patients, as well as purpose of each phase of the process. Some CF modulator fun facts have also been added so you can compare CF drug development vs. what the average drug goes through in the United States.
It really can’t be overstated how much blood, sweat and tears go in to drug development. Most of the general population doesn’t get the chance to participate in the incredible experience that is a clinical trial, but in the cystic fibrosis community, many of us are able to participate in several throughout our lifetime.
But what exactly goes on during drug development? Let’s check it out. Please keep in mind that facts around Kalydeco, Orkambi, Symdeko and a potential triple combo therapy are approximate and are presented to show the details of how each clinical trial stage applies in a real-world scenario to our CF community. It really is astounding to see how far we have come – and it’s not hard to get excited about where we are going, quickly!
Keep in mind that each stage of clinical trials requires expert scientists, top-notch research equipment, labs in which to conduct research; personnel to design trials, analyze trial results, and to meet with the FDA many times to discuss safety, trial progress and trial design; clinical trial sites across the globe, medical personnel to conduct clinical trials, internal review boards to protect patient safety at each study site, and patients to actually participate in each trial.
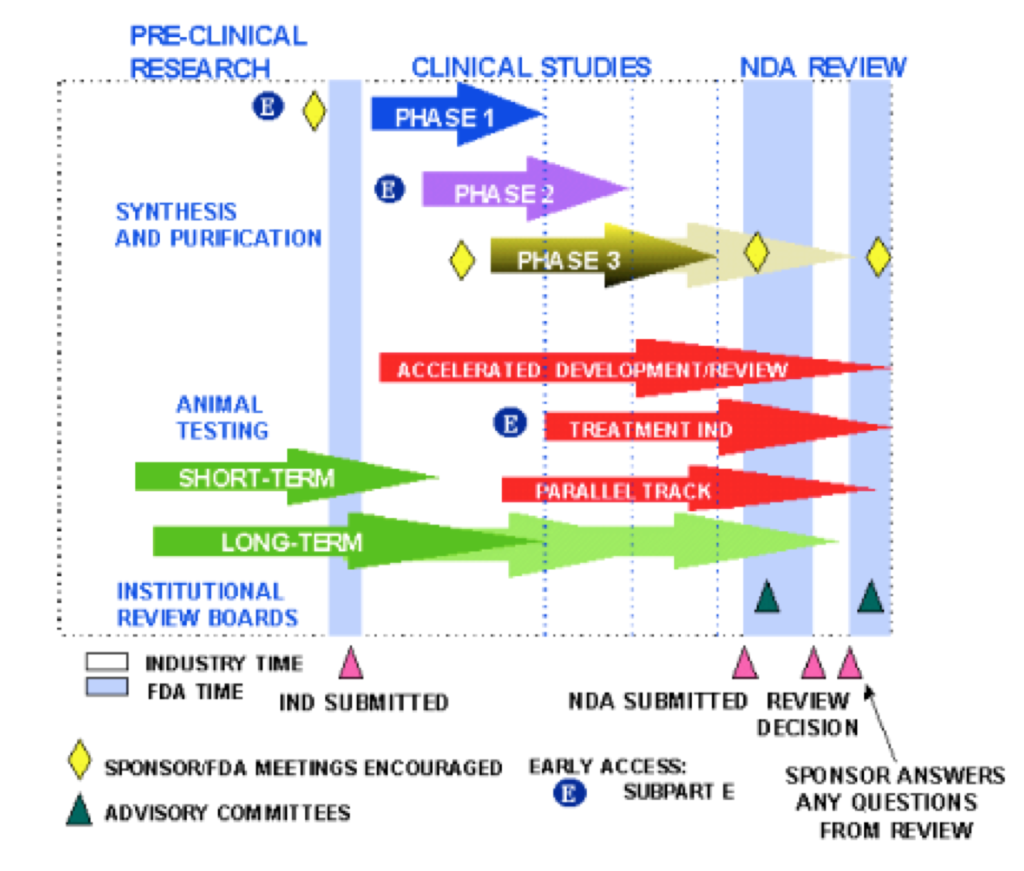
Pre-Clinical Stage
- Purpose – Laboratory testing and animal testing that is done before a drug is tested in humans
- Cost – 8% of total research and development costs occur during this pre-clinical phase, which represents $1.098 billion capitalized on average. With cystic fibrosis drugs and the Cystic Fibrosis Foundation, for a drug “to be listed as a preclinical program on the pipeline, a program must have received an award from the Foundation committing at least $2 million or have received an award that includes payments that depend on the drug reaching clinical trials.”
- Time – Typically takes on average 2 months from synthesis to entering in to Phase 1 clinical trial (1st phase of human trials)
Phase 1 Study
- Purpose – To test the new medication on usually healthy people – but sometimes those with the disease that is being studied. The trial typically has around 20-80 participants with the goal of understanding safety, side effects and correct dosage of the drug
- Cost – From Phase I clinical trials all the way through the process to approval of a new drug, average cost is $1.46 billion
- Time – Study length is typically a few months
- Success rate – In this particular study by the Tuft’s Center for Drug Development of drugs from 1995-2007, 11.83% of drugs made it from Phase 1 to FDA approval. It’s amazing to think of Kalydeco, Orkambi and Symdeko in that context – all 3 of those drugs had less than a 12% chance of making it to the market; yet defied all odds and are availble in the United States for patients with CF. In addition, 60% of drugs made it from Phase 1 to Phase 2
Of interest, Kalydeco was in Phase I trials for approximately 2 quarters in 2006. Cystic Fibrosis Foundation Therapeutics (CFFT) paid $13.3 million (money raised by patients and families in the American CF community) to fund studies for Kalydeco. Up until 2006, the CFFT paid Vertex $40 million to develop medications for cystic fibrosis
Vertex opted to study 4 next generation correctors in Phase I trials that would be included in the “triple combo” aimed at heterozygous DF508 patients. VX-659, one of the components of one of the triple combo Phase III trials currently underway, began in late 2016 and was completed July 2017.
Phase 2 Study
- Purpose – Usually 100 – 300 patients and looks at dosing, efficacy and safety on patients with the disease the drug is trying to treat
- Time – Study length can be several months to 2 years. From Phase 2 to Phase 3, on average it takes 3 months
- Success rate – 35.5% of drugs move from Phase 2 to Phase 3
Of note, Vertex also decided to study and fund 4 “next generation” correctors in Phase II trials to be included in the so-called triple combo– VX-445 and VX-659 Phase II studies began August 2017 and by January 2018, results were in from these studies and Vertex decided to advance only VX-445 and VX-659 to Phase III trials.
Phase 3 Study
- Purpose – To examine safety, efficacy, and sometimes different doses of the study drug. Several hundred patients to several thousand typically participate in these trials
- Time – Study is typically 1-4 years. From Phase 3 to New Drug Application it’s 7 months on average
- Success rate – 62% make it from Phase 3 to New Drug Application submission to the FDA
With continued lightning speed, Vertex initiated VX-659 and VX-445 Phase III clinical trials in February and March 2018 respectively for CF patients that are heterozygous for DF508.
FDA Submission (also called New Drug Application – or NDA- for small molecules and Biologics License Application – or BLA- for large molecules)
- Time – From NDA submission to approval is 16 months
On October 2011 Vertex submitted an NDA to the FDA for Kalydeco. On January 31, 2012, Kalydeco became the first FDA approved medication to treat the underlying cause of cystic fibrosis . Kalydeco also become one of the fastest FDA approvals in history, thanks in part to The Orphan Drug Act.
The triple combo therapy from Vertex, if FDA approved by 2020, may have gone through Phase I to FDA approval in around 4 years – almost unheard of in drug development.
Phase 4
- Purpose – After FDA approval, sometimes the FDA requires monitoring of safety and efficacy of the drug in the general population. On occasion, safety issues aren’t seen until the drug is used in a larger population over a longer period of time
In summary, it takes on average 128 months (10+ years) and $2.6 billion to bring a new medication to the market, with a large cost incurred during clinical trials.
For every 8.5 drugs that enter into human trials, only 1 will be FDA approved. It simply cannot be overstated how risky drug development is; how high failure rate is; and how extremely fortunate we as a CF community are to have Kalydeco, Orkambi and Symdeko.
The next time you take Kalydeco, Orkambi, Symdeko or a clinical trial medication for cystic fibrosis, please think of all the hard work that has gone in to the drug you are benefitting from. The scientists, the researchers, the clinicians, the clinical trial participants, the American families that fundraised hundreds of millions of dollars since the 1950s, and the Cystic Fibrosis Foundation volunteers and personnel who made these drugs possible.
From “The Antidote” by Barry Werth: Kalydeco [and of course down the line, Orkambi and Symdeko] was the result of “…a decade of all-out heterodoxy and entrepreneurial risk taking: [Dr. Beall, then president and CEO of the American Cystic Fibrosis Foundation, his] determination to push venture philanthropy into for-profit research; his refusal to yield to established opinion that CF drugs would treat only the symptoms of the disease but not its cause; his insistence on going after CFTR… his willingness to foot part of the bill for Vertex’s clinical development.”
Here’s to many more cystic fibrosis medications to come!
The views expressed on any guest column [Drug Development Wednesday], are that of guest contributors, and not necessarily those of Gunnar Esiason or the Boomer Esiason Foundation. Nothing in guest columns should be considered medical advice; such advice can only be given by a physician who is experienced with cystic fibrosis. The Boomer Esiason Foundation, Gunnar Esiason, and guests cannot be held responsible for any damage which may result from using the information on this website without the permission of your medical doctor.